Injected drug, lecanemab, slowed the progress of the brain-wasting disease by 27 percent compared to a placebo, meeting the study’s main goal, say drugmakers Eisai and Biogen.
An experimental Alzheimer’s drug
developed by Eisai Co Ltd and Biogen Inc
significantly slowed the cognitive and functional decline in a large
trial of patients in the early stages of the disease, the
companies have said.
The injected drug, lecanemab, slowed the progress of the
brain-wasting disease by 27 percent compared to a placebo, meeting the
study’s main goal, and offering an apparent win for the
companies and potentially for patients and their families
desperate for an effective treatment.
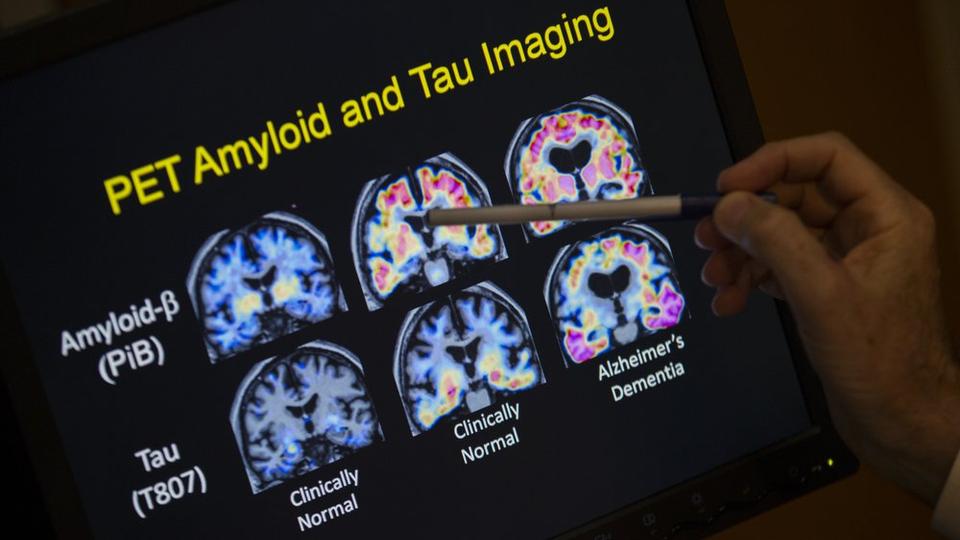
Eisai, on Tuesday, said the results from the 1,800-patient trial prove the longstanding theory that the removal of sticky deposits of a protein called amyloid beta from the brains of people with early Alzheimer’s can delay advance of the debilitating disease.
Analysts, such as Salim Syed at Mizuho Securities, have said
the results would be considered a “win” if lecanemab slowed the
rate of decline by around 25 percent, and that shares of both companies
could jump on the news.
Lecanemab, like the companies’ previous drug Aduhelm, is an
antibody designed to remove those amyloid deposits. Unlike
Aduhelm, lecanemab targets forms of amyloid that have not yet
clumped together.
The so-called amyloid hypothesis has been challenged by some
scientists, particularly after the US Food and Drug
Administration’s controversial approval of Aduhelm in 2021 based
on its plaque-clearing ability rather than proof that it helped
slow cognitive decline. The decision came after the FDA’s own
panel of outside experts had advised against approval.
READ MORE: Flu vaccine can reduce risk of Alzheimer’s disease by 40 percent – study
Pending FDA approval
Aduhelm was the first new Alzheimer’s drug approved in 20
years after a long list of high-profile failures for the
industry.
Eisai, leader of the 50-50 partnership’s lecanemab programme,
is seeking FDA approval under the same accelerated pathway as
Aduhelm, with a decision expected in early January. But on
Tuesday the Japanese drugmaker said it will use the new efficacy
results to submit lecanemab for traditional FDA review.
The company said it will also seek authorisation in Japan
and Europe during its current fiscal year, ending March 31.
The Phase III trial evaluated the drug’s ability to reduce
cognitive and functional decline based on the Clinical Dementia
Rating-Sum of Boxes (CDR-SB), a numerical scale used to quantify
the severity of dementia in patients in areas such as memory,
orientation, judgment and problem solving and personal care.
Aduhelm’s approval was a rare bright spot for Alzheimer’s
patients, but critics have called for more evidence that
amyloid-targeting drugs are worth the cost.
But Medicare, the US government health plan for people 65
and older, this year said it would only pay for Aduhelm if
patients were enrolled in a valid clinical trial, which sharply
curtailed the medication’s use. Since Alzheimer’s is a disease
of aging, an estimated 85 percent of patients eligible for the drug are
covered by the government plan.
The number of Americans living with Alzheimer’s is expected
to rise to around 13 million by 2050 from more than 6 million
currently, according to the Alzheimer’s Association. Globally,
that figure could rise to 139 million by 2050 without an
effective treatment, according to Alzheimer’s Disease
International.
Other plaque-targeting antibodies in late-stage development
for Alzheimer’s patients include Roche Holding AG’s
gantenerumab and Eli Lilly and Co’s donanemab.
READ MORE: Physically active older adults may steer clear of Alzheimer’s disease
Source: Reuters
Alzheimer’s drug succeeds in ‘slowing’ cognitive decline
Source: News Achor Trending
0 Comments